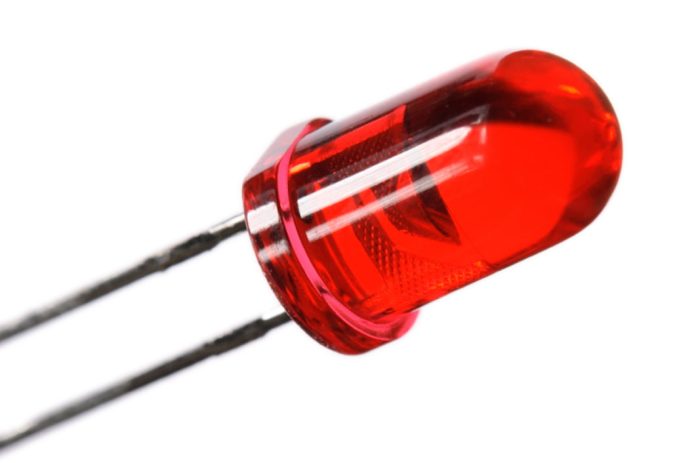
Q: Could you explain why LED light sources are so much more economical to run than incandescent lights? I’m also interested in whether it’s best to use silicon or gallium arsenide-type LED lights for infrared lighting. Which is more economical and delivers more red light?
A: LEDs are PN junction diodes – pure and simple. The reason they’re so efficient is that they convert an electrical current directly into light. There are both silicon and gallium arsenide LEDs, with silicon types putting out a small amount of near infrared, including heat, and gallium arsenide producing large amounts of red and near infrared, as well as visible light. It’s gallium arsenide that’s most commonly used for light sources.
Something else to bear in mind is that these are not the only possible LED types. Most LEDs are typically compound junction gallium arsenide, but there are LEDs made of aluminium gallium arsenide, gallium phosphide, gallium arsenide phosphide, indium gallium nitride, silicon carbide, aluminium gallium indium nitride and more. Depending on construction, light is emitted at wavelengths of between 400 and 800nm.
With gallium arsenide LEDs, the voltage threshold to produce a forward bias across a PN diode is 1.3V and this little stream of electricity excites electrons as they leap across the PN junction causing each to emit a photon. A typical LED luminary incorporates an array of these diodes, but this LED array is still much more efficient than incandescent lamps, which can draw 100W or more and lose more than half their energy to heat.
The reason for this is that an incandescent bulb is a resistive element – a bit like a toaster or a stove element – and when current slows down (as it does when it’s squeezing through a tungsten filament or less conductive metal) things warm up.
#sen.news